Studying Switchable Solvents
This article is a modified version of an article written by Matt Shipman, Research Lead in University Communications.
Most chemical manufacturing operations utilize significant amounts of liquid solvents. Examples are solvents that carry dissolved or suspended constituents while flowing in a pipe, or that catalyze chemical reactions, or that are used to separate products, by-products and unused reactants as they exit from a chemical reactor, to name a few. Purifying and recycling contaminated solvent for reuse is oftentimes difficult, hazardous, energy-intensive and expensive.
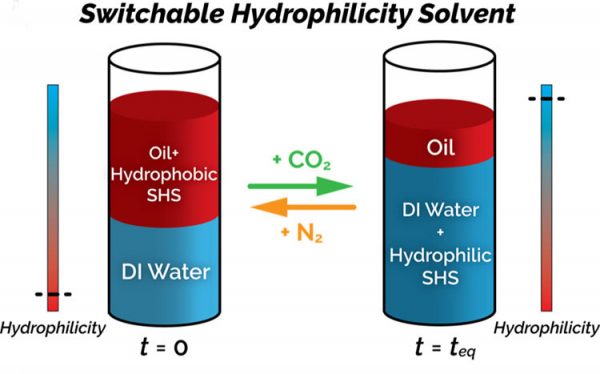
Switchable solvents are solvents that reversibly change physical properties abruptly. This unique property is a consequence of a reversible reaction in response to an external stimulus such as a temperature change and/or the addition or removal of a gas. Because of the reversibility of the reaction, the changed solvent can easily be brought back to its original state.
From a green engineering perspective, using switchable solvents to reduce energy usage and reduce the use of hazardous materials in a chemical plant is highly desirable. Doing that requires discovering new switchable solvents, quantifying their properties, and developing processes that take full advantage of their uniqueness.
Professor Milad Abolhasani and his colleagues have demonstrated a new, green technology for both accelerated screening and retrieving switchable solvents used in green chemistry applications. The new approach makes the screening process hundreds of times faster and drastically accelerates the rate at which solvents can be retrieved from solution.
“We have effectively created a platform that makes green chemistry greener,” says Prof. Abolhasani, the corresponding author of a paper on the work. “This work expedites industry’s ability to identify the best switchable solvent for a specific chemical process and then gives industry advanced tools to retrieve that solvent far more quickly than is possible using previous approaches.”
In this case, the switchable solvents change their physicochemical properties when exposed to carbon dioxide (CO2). This study focused on solvents that become hydrophilic (miscible with water) in the presence of CO2 and water, but are hydrophobic (immiscible with water) when CO2 is removed. This makes them attractive for use in chemical and pharmaceutical industry processes, because the solvent can be easily removed from the product by adding CO2 and water. The solvent can then be reclaimed from the water by removing the CO2.
“However, from an industrial point of view, there are significant challenges,” Abolhasani says. “Specifically, the process for screening candidates to identify the most efficient switchable solvent for a particular application can be extremely time- and labor-intensive. And once you have the right switchable solvent candidate, removing it on a large scale can also take a long time.”
To address the screening problem, Abolhasani’s team made use of a microscale flow chemistry platform that runs 5-microliter samples through a gas-permeable tube that is surrounded by CO2. This ensures the solvent in the sample is in constant contact with the CO2 (i.e., intensified mass transfer), accelerating the reaction and the solvent recovery process.
Using this technique, the researchers can determine a solvent’s efficiency, using image processing, in as little as three minutes. The platform also allows them to run multiple samples simultaneously. Accounting for the time needed to prepare each sample, the system allows users to run approximately 280 screening experiments per day.
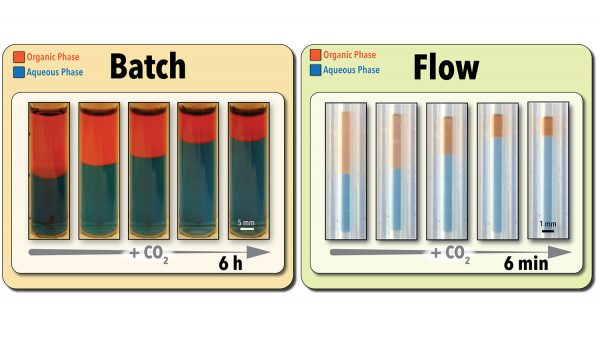
By comparison, conventional batch testing techniques require the use of larger sample sizes. For example, testing the efficiency of a 5-milliliter sample using conventional batch techniques takes between six and eight hours – or approximately one test per day.
Abolhasani’s team also demonstrated in experimental testing that the flow chemistry technique was not only faster, but was just as accurate as conventional batch testing at determining a solvent’s efficiency.
In addition, the researchers have recently shown that they can reconfigure the same flow chemistry platform utilized for rapid switchable solvent screening into a continuous flow mode for retrieving solvents on a large scale. “Our approach is also more cost effective in that it is completely computer-controlled and is, therefore, less labor-intensive,” Abolhasani says.
“We’re excited about the potential of this process intensification technology and are looking for partners to help us transfer the technique from the lab to industrial R&D and manufacturing applications.”
The paper, “Accelerated Material-Efficient Investigation of Switchable Hydrophilicity Solvents for Energy-Efficient Solvent Recovery,” is published in the journal ACS Sustainable Chemistry & Engineering. First author of the paper is Suyong Han, a Ph.D. student Ph.D. in the Abolhasani research group. The paper was co-authored by Keshav Raghuvanshi, a postdoctoral researcher Prof. Abolhasani’s group. The work was done with support from the American Chemical Society Petroleum Research Fund, under grant number 59602-DNI9.